MIL-53
MIL-53 (MIL ⇒ Matériaux de l′Institut Lavoisier) ist eine sehr bekannte und gut untersuchte Struktur der Materialklasse der Metall-organischen Gerüstverbindungen (MOFs). Sie wurde von der Arbeitsgruppe von Gérard Férey am Institut Lavoisier der Universität Versailles-Saint-Quentin-en-Yvelines hergestellt.[1] Man unterscheidet MIL-53as (as = as synthesized, mit Einschlüssen von Terephthalsäure), MIL-53lt (lt = low temperature, die Tieftemperaturmodifikation) und MIL-53ht (ht = high temperature, die Hochtemperaturmodifikation).
| Kristallstruktur | |||||||
|---|---|---|---|---|---|---|---|
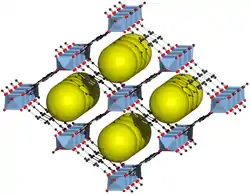 | |||||||
| grau: Al3+, rot: O2−, schwarz: C | |||||||
| Allgemeines | |||||||
| Name | MIL-53 | ||||||
| Andere Namen |
| ||||||
| Verhältnisformel | C8H5AlO5 | ||||||
| Externe Identifikatoren/Datenbanken | |||||||
| |||||||
| Eigenschaften | |||||||
| Molare Masse | 208,10 g·mol−1 | ||||||
| Aggregatzustand |
fest | ||||||
| Schmelzpunkt |
Zersetzung ab 500 °C[1] | ||||||
| Sicherheitshinweise | |||||||
| |||||||
| Soweit möglich und gebräuchlich, werden SI-Einheiten verwendet. Wenn nicht anders vermerkt, gelten die angegebenen Daten bei Standardbedingungen. | |||||||
Bekannte Strukturanaloga
Die MIL-53-Struktur wurde mit verschiedenen Metallen synthetisiert, wobei überwiegend dreiwertige Metalle und seltener zwei- oder vierwertige Metalle verwendet wurden.[3]
| Bezeichnung | Metallzentrum und
Oxidationszustand |
Jahr der Erstpublikation | Alternativer Name | Zitation |
|---|---|---|---|---|
| MIL-53(V) | V3+ | 2002 | MIL-47 | [4][5] |
| V4+ | ||||
| MIL-53(Cr) | Cr3+ | 2002 | [6][7] | |
| MIL-53(Al) | Al3+ | 2004 | [1] | |
| MIL-53(Fe) | Fe3+ | 2005 | [8] | |
| Fe2+ | 2005 | [8] | ||
| MIL-53(In) | In3+ | 2005 | [9] | |
| MIL-53(Co) | Co2+ | 2005 | MOF-71 | [10][11] |
| MIL-53(Ga) | Ga3+ | 2008 | [12] | |
| MIL-53(Mn) | Mn2+ | 2010 | [13] | |
| MIL-53(Sc) | Sc3+ | 2011 | [14] | |
| MIL-53(Ni) | Ni2+ | 2013 | [11] |
Es können nicht nur verschiedene Metalle, sondern auch verschiedene Derivate der Terephthalsäure als Linkermoleküle verwendet werden um MIL-53-Strukturen herzustellen. Diese Linkermoleküle besitzen zusätzlich zu den zwei Carboxylatgruppen meistens eine oder mehrere zusätzliche funktionelle Gruppen am Benzolring, welche nicht für den Aufbau der Gerüststruktur verwendet werden.
| Funktioneller Linker | Metallzentrum (M) | |||||
|---|---|---|---|---|---|---|
| V | Cr | Al | Fe | In | Ga | |

|
[15] | - | [16][17] | [18] | [19] | [19] |
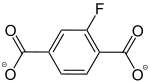
2-Fluorobenzol-1,4-dicarboxylat |
[20] | - | [21] | - | - | - |
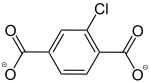
2-Chlorobenzol-1,4-dicarboxylat |
[22] | [23] | [24] | [25] | - | - |
|
[26] | - | [27] | [28] | [29] | - |
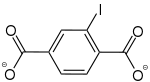
2-Iodobenzol-1,4-dicarboxylat |
- | - | [30] | - | - | - |
|
- | - | [27] | - | [29] | - |
Benzol-1,2,4-tricarboxylat |
- | - | [31] | - | - | - |
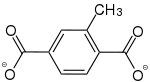
2-Methylbenzol-1,4-dicarboxylat |
[22] | [23] | [24] | [25] | - | - |
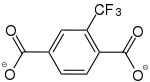
2-Trifluormethylbenzol-1,4-dicarboxylat |
[32] | - | - | - | - | - |
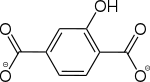
2-Hydroxybenzol-1,4-dicarboxylat |
[22] | - | [33] | - | - | - |
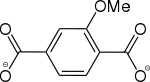
2-Methoxybenzol-1,4-dicarboxylat |
[32] | - | - | - | - | - |
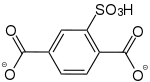
2-Sulfonsäurebenzol-1,4-dicarboxylat |
- | - | [34] | - | - | - |
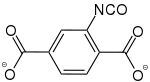
2-Isocyanatbenzol-1,4-dicarboxylat |
- | - | [35] | - | - | - |
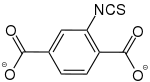
2-Isothiocyanatbenzol-1,4-dicarboxylat |
- | - | [35] | - | - | - |
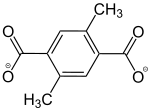
2,5-Dimethylbenzol-1,4-dicarboxylat |
[36] | - | - | - | - | - |

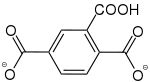
|
[37] | - | [27] | [25] | [29] | - |
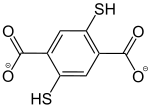
2,5-Dithiolbenzol-1,4-dicarboxylat |
- | - | [38] | - | - | - |
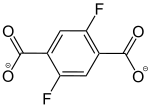
2,5-Difluorobenzol-1,4-dicarboxylat |
[39] | - | [40] | - | - | - |
benzene-1%252C4-dicarboxylate.svg.png.webp)
2,5-Bis(trifluormethyl)benzol-1,4-dicarboxylat |
[41] | - | - | [25] | - | - |
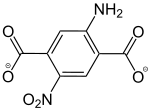
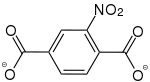
2-Amino-5-nitrobenzol-1,4-dicarboxylat |
- | - | [42] | - | [42] | [42] |

Benzol-1,2,4,5-tetracarboxylat |
- | - | [43]
MIL-121 |
[44]MIL-82 | - | - |
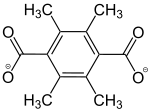
2,3,5,6-tetramethylbenzol-1,4-dicarboxylat |
- | [45]
MIL-105 |
- | - | - | - |
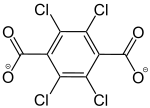
2,3,5,6-Tetrachlorobenzol-1,4-dicarboxylat |
[36] | - | - | - | - | - |
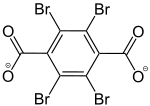
2,3,5,6-Tetrabromobenzol-1,4-dicarboxylat |
[36] | - | - | - | - | - |
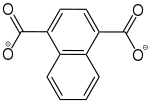
Naphthalen-1,4-dicarboxylat |
[46] | - | [47] | - | - | - |
Synthese
MIL-53 kann durch eine Hydrothermalsynthese ausgehend von Aluminiumnitrat und Terephthalsäure in Wasser im molaren Verhältnis 1:0,5:80 bei 180 °C erhalten werden. Die in den Poren eingeschlossene Terephthalsäure der as-Form kann durch Sublimation entfernt werden. Bei 500 K liegt ausschließlich die ht-Form vor.[1]
Eigenschaften
Alle Formen von MIL-53 weisen die gleiche Netzwerkstruktur auf. Eindimensionale parallele Ketten aus [AlO4(OH)2]-Oktaedern werden durch die Terephthal-Linker zu einem dreidimensionalen Netzwerk verbunden. Die Carboxylat-Gruppen koordinieren die [Al(O4)(OH)2]-Oktaeder verbrückend, wobei diese zusätzlich durch die OH-Gruppen Eckenverknüpft sind. Dazwischen befinden sich bis zu 8,5 Å große Poren.[1] Beim Erhitzen geht MIL-53 eine reversible Strukturänderung von einer offenporigen in eine geschlossenporige Struktur ein. Diese zeigt ein Hysterese-Verhalten, der Übergang erfolgt beim Erwärmen schon bei 125–150 K, beim Abkühlen erst bei 325–375 K.[48] Die Hochtemperaturmodifikation sowie MIL-53as kristallisieren im orthorhombischen Kristallsystem, während die Tieftemperaturmodifikation im monoklinen Kristallsystem vorliegt.[1][6]
MIL-53 ist chemisch sehr viel beständiger als die meisten anderen MOFs. Die Verbindung wird weder durch Luft oder Wasser zerstört und ist thermisch bis 500 °C stabil.[1]
MIL-53 kann verschiedene Gase wie Kohlenstoffdioxid, Wasser, Wasserstoff oder Methan adsorbieren. Auf Grund der Flexibilität des Netzwerkes kann sich dieses bei der Aufnahme von Kohlenstoffdioxid oder Wasser verändern, man spricht dabei von einer Art "Atmen".[49]
Einzelnachweise
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille, G. Férey: A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration. In: Chem. Eur. J. 2004, 10, S. 1373–1382, doi:10.1002/chem.200305413.
- Dieser Stoff wurde in Bezug auf seine Gefährlichkeit entweder noch nicht eingestuft oder eine verlässliche und zitierfähige Quelle hierzu wurde noch nicht gefunden.
- Franck Millange, Richard I. Walton: MIL-53 and its Isoreticular Analogues: a Review of the Chemistry and Structure of a Prototypical Flexible Metal-Organic Framework. In: Israel Journal of Chemistry. Band 58, Nr. 9-10, Oktober 2018, S. 1019–1035, doi:10.1002/ijch.201800084 (wiley.com [abgerufen am 7. April 2020]).
- Karin Barthelet, Jérôme Marrot, Didier Riou, Gérard Férey: A Breathing Hybrid Organic–Inorganic Solid with Very Large Pores and High Magnetic Characteristics. In: Angewandte Chemie International Edition. Band 41, Nr. 2, 2002, ISSN 1521-3773, S. 281–284, doi:10.1002/1521-3773(20020118)41:23.0.CO;2-Y.
- Hervé Leclerc, Thomas Devic, Sabine Devautour-Vinot, Philippe Bazin, Nathalie Audebrand: Influence of the Oxidation State of the Metal Center on the Flexibility and Adsorption Properties of a Porous Metal Organic Framework: MIL-47(V). In: The Journal of Physical Chemistry C. Band 115, Nr. 40, 13. Oktober 2011, ISSN 1932-7447, S. 19828–19840, doi:10.1021/jp206655y.
- C. Serre, F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, D. Louër, and G. Férey: Very Large Breathing Effect in the First Nanoporous Chromium(III)-Based Solids: MIL-53 or CrIII(OH)·{O2C-C6H4-CO2}·{HO2C-C6H4-CO2H}x·H2Oy. In: J. Am. Chem. Soc. 2002, 124, 45, S. 13519–13526, doi:10.1021/ja0276974.
- Franck Millange, Christian Serre, Gérard Férey: Synthesis, structure determination and properties of MIL-53as and MIL-53ht: the first Criii hybrid inorganic–organic microporous solids: Criii(OH)·{O2C–C6H4–CO2}·{HO2C–C6H4–CO2H}xElectronic supplementary information (ESI) available: crystal data, atomic coordinates and metrical parameters for MIL-53as and MIL-53ht. See http://www.rsc.org/suppdata/cc/b2/b201381a/. In: Chemical Communications. Nr. 8, 11. April 2002, S. 822–823, doi:10.1039/b201381a.
- Tabatha R. Whitfield, Xiqu Wang, Lumei Liu, Allan J. Jacobson: Metal-organic frameworks based on iron oxide octahedral chains connected by benzenedicarboxylate dianions. In: Solid State Sciences. Band 7, Nr. 9, September 2005, S. 1096–1103, doi:10.1016/j.solidstatesciences.2005.03.007.
- Ekaterina V. Anokhina, Marie Vougo-Zanda, Xiqu Wang, Allan J. Jacobson: In(OH)BDC·0.75BDCH 2 (BDC = Benzenedicarboxylate), a Hybrid Inorganic−Organic Vernier Structure. In: Journal of the American Chemical Society. Band 127, Nr. 43, November 2005, ISSN 0002-7863, S. 15000–15001, doi:10.1021/ja055757a.
- Nathaniel L. Rosi, Jaheon Kim, Mohamed Eddaoudi, Banglin Chen, Michael O'Keeffe: Rod Packings and Metal−Organic Frameworks Constructed from Rod-Shaped Secondary Building Units. In: Journal of the American Chemical Society. Band 127, Nr. 5, Februar 2005, ISSN 0002-7863, S. 1504–1518, doi:10.1021/ja045123o.
- Alexis S. Munn, Guy J. Clarkson, Franck Millange, Yves Dumont, Richard I. Walton: M(ii) (M = Mn, Co, Ni) variants of the MIL-53-type structure with pyridine-N-oxide as a co-ligand. In: CrystEngComm. Band 15, Nr. 45, 2013, ISSN 1466-8033, S. 9679, doi:10.1039/c3ce41268g.
- Marie Vougo-Zanda, Jin Huang, Ekaterina Anokhina, Xiqu Wang, Allan J. Jacobson: Tossing and Turning: Guests in the Flexible Frameworks of Metal(III) Dicarboxylates. In: Inorganic Chemistry. Band 47, Nr. 24, 15. Dezember 2008, ISSN 0020-1669, S. 11535–11542, doi:10.1021/ic800008f.
- Guohai Xu, Xiaoguang Zhang, Peng Guo, Chengling Pan, Hongjie Zhang: Mn II -based MIL-53 Analogues: Synthesis Using Neutral Bridging μ 2 -Ligands and Application in Liquid-Phase Adsorption and Separation of C6−C8 Aromatics. In: Journal of the American Chemical Society. Band 132, Nr. 11, 24. März 2010, ISSN 0002-7863, S. 3656–3657, doi:10.1021/ja910818a.
- John P.S. Mowat, Stuart R. Miller, Alexandra M.Z. Slawin, Valerie R. Seymour, Sharon E. Ashbrook: Synthesis, characterisation and adsorption properties of microporous scandium carboxylates with rigid and flexible frameworks. In: Microporous and Mesoporous Materials. Band 142, Nr. 1, Juni 2011, S. 322–333, doi:10.1016/j.micromeso.2010.12.016.
- Karen Leus, Sarah Couck, Matthias Vandichel, Gauthier Vanhaelewyn, Ying-Ya Liu: Synthesis, characterization and sorption properties of NH2-MIL-47. In: Physical Chemistry Chemical Physics. Band 14, Nr. 44, 2012, ISSN 1463-9076, S. 15562, doi:10.1039/c2cp42137b.
- Tim Ahnfeldt, Daniel Gunzelmann, Thierry Loiseau, Dunja Hirsemann, Jürgen Senker: Synthesis and Modification of a Functionalized 3D Open-Framework Structure with MIL-53 Topology. In: Inorganic Chemistry. Band 48, Nr. 7, 6. April 2009, ISSN 0020-1669, S. 3057–3064, doi:10.1021/ic8023265.
- Tim Ahnfeldt, Nathalie Guillou, Daniel Gunzelmann, Irene Margiolaki, Thierry Loiseau: [Al 4 (OH) 2 (OCH 3 ) 4 (H 2 N-bdc) 3 ]⋅ x H 2 O: A 12-Connected Porous Metal-Organic Framework with an Unprecedented Aluminum-Containing Brick. In: Angewandte Chemie International Edition. Band 48, Nr. 28, 29. Juni 2009, S. 5163–5166, doi:10.1002/anie.200901409.
- Sebastian Bauer, Christian Serre, Thomas Devic, Patricia Horcajada, Jérôme Marrot: High-Throughput Assisted Rationalization of the Formation of Metal Organic Frameworks in the Iron(III) Aminoterephthalate Solvothermal System. In: Inorganic Chemistry. Band 47, Nr. 17, September 2008, ISSN 0020-1669, S. 7568–7576, doi:10.1021/ic800538r.
- Pablo Serra-Crespo, Elena Gobechiya, Enrique V. Ramos-Fernandez, Jana Juan-Alcañiz, Alberto Martinez-Joaristi: Interplay of Metal Node and Amine Functionality in NH 2 -MIL-53: Modulating Breathing Behavior through Intra-framework Interactions. In: Langmuir. Band 28, Nr. 35, 4. September 2012, ISSN 0743-7463, S. 12916–12922, doi:10.1021/la302824j.
- Shyam Biswas, Tom Rémy, Sarah Couck, Dmytro Denysenko, Geert Rampelberg: Partially fluorinated MIL-47 and Al-MIL-53 frameworks: influence of functionalization on sorption and breathing properties. In: Physical Chemistry Chemical Physics. Band 15, Nr. 10, 2013, ISSN 1463-9076, S. 3552, doi:10.1039/c3cp44204g.
- Shyam Biswas, Tom Rémy, Sarah Couck, Dmytro Denysenko, Geert Rampelberg: Partially fluorinated MIL-47 and Al-MIL-53 frameworks: influence of functionalization on sorption and breathing properties. In: Physical Chemistry Chemical Physics. Band 15, Nr. 10, 2013, ISSN 1463-9076, S. 3552, doi:10.1039/c3cp44204g.
- Shyam Biswas, Danny E. P. Vanpoucke, Toon Verstraelen, Matthias Vandichel, Sarah Couck: New Functionalized Metal–Organic Frameworks MIL-47-X (X = −Cl, −Br, −CH 3 , −CF 3 , −OH, −OCH 3 ): Synthesis, Characterization, and CO 2 Adsorption Properties. In: The Journal of Physical Chemistry C. Band 117, Nr. 44, 7. November 2013, ISSN 1932-7447, S. 22784–22796, doi:10.1021/jp406835n.
- Pascal G. Yot, Ke Yang, Vincent Guillerm, Florence Ragon, Vladimir Dmitriev: Impact of the Metal Centre and Functionalization on the Mechanical Behaviour of MIL-53 Metal-Organic Frameworks: Impact of the Metal Centre and Functionalization on the Mechanical Behaviour of MIL-53 Metal-Organic Frameworks. In: European Journal of Inorganic Chemistry. Band 2016, Nr. 27, September 2016, S. 4424–4429, doi:10.1002/ejic.201600263.
- Shyam Biswas, Tim Ahnfeldt, Norbert Stock: New Functionalized Flexible Al-MIL-53-X (X = -Cl, -Br, -CH 3 , -NO 2 , -(OH) 2 ) Solids: Syntheses, Characterization, Sorption, and Breathing Behavior. In: Inorganic Chemistry. Band 50, Nr. 19, 3. Oktober 2011, ISSN 0020-1669, S. 9518–9526, doi:10.1021/ic201219g.
- Thomas Devic, Patricia Horcajada, Christian Serre, Fabrice Salles, Guillaume Maurin: Functionalization in Flexible Porous Solids: Effects on the Pore Opening and the Host−Guest Interactions. In: Journal of the American Chemical Society. Band 132, Nr. 3, 27. Januar 2010, ISSN 0002-7863, S. 1127–1136, doi:10.1021/ja9092715.
- Shyam Biswas, Danny E. P. Vanpoucke, Toon Verstraelen, Matthias Vandichel, Sarah Couck: New Functionalized Metal–Organic Frameworks MIL-47-X (X = −Cl, −Br, −CH 3 , −CF 3 , −OH, −OCH 3 ): Synthesis, Characterization, and CO 2 Adsorption Properties. In: The Journal of Physical Chemistry C. Band 117, Nr. 44, 7. November 2013, ISSN 1932-7447, S. 22784–22796, doi:10.1021/jp406835n.
- Shyam Biswas, Tim Ahnfeldt, Norbert Stock: New Functionalized Flexible Al-MIL-53-X (X = -Cl, -Br, -CH 3 , -NO 2 , -(OH) 2 ) Solids: Syntheses, Characterization, Sorption, and Breathing Behavior. In: Inorganic Chemistry. Band 50, Nr. 19, 3. Oktober 2011, ISSN 0020-1669, S. 9518–9526, doi:10.1021/ic201219g.
- Thomas Devic, Patricia Horcajada, Christian Serre, Fabrice Salles, Guillaume Maurin: Functionalization in Flexible Porous Solids: Effects on the Pore Opening and the Host−Guest Interactions. In: Journal of the American Chemical Society. Band 132, Nr. 3, 27. Januar 2010, ISSN 0002-7863, S. 1127–1136, doi:10.1021/ja9092715.
- Lei Wu, Gérald Chaplais, Ming Xue, Shilun Qiu, Joël Patarin: New functionalized MIL-53(In) solids: syntheses, characterization, sorption, and structural flexibility. In: RSC Advances. Band 9, Nr. 4, 2019, ISSN 2046-2069, S. 1918–1928, doi:10.1039/C8RA08522F.
- Babak Tahmouresilerd, Patrick J. Larson, Daniel K. Unruh, Anthony F. Cozzolino: Make room for iodine: systematic pore tuning of multivariate metal–organic frameworks for the catalytic oxidation of hydroquinones using hypervalent iodine. In: Catalysis Science & Technology. Band 8, Nr. 17, 2018, ISSN 2044-4753, S. 4349–4357, doi:10.1039/C8CY00794B.
- Nele Reimer, Barbara Gil, Bartosz Marszalek, Norbert Stock: Thermal post-synthetic modification of Al-MIL-53–COOH: systematic investigation of the decarboxylation and condensation reaction. In: CrystEngComm. Band 14, Nr. 12, 2012, ISSN 1466-8033, S. 4119, doi:10.1039/c2ce06649a.
- Shyam Biswas, Danny E. P. Vanpoucke, Toon Verstraelen, Matthias Vandichel, Sarah Couck: New Functionalized Metal–Organic Frameworks MIL-47-X (X = −Cl, −Br, −CH 3 , −CF 3 , −OH, −OCH 3 ): Synthesis, Characterization, and CO 2 Adsorption Properties. In: The Journal of Physical Chemistry C. Band 117, Nr. 44, 7. November 2013, ISSN 1932-7447, S. 22784–22796, doi:10.1021/jp406835n.
- Dieter Himsl, Dirk Wallacher, Martin Hartmann: Improving the Hydrogen-Adsorption Properties of a Hydroxy-Modified MIL-53(Al) Structural Analogue by Lithium Doping. In: Angewandte Chemie International Edition. Band 48, Nr. 25, 8. Juni 2009, S. 4639–4642, doi:10.1002/anie.200806203.
- Jinzhu Chen, Kegui Li, Limin Chen, Ruliang Liu, Xing Huang: Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal–organic frameworks. In: Green Chem. Band 16, Nr. 5, 2014, ISSN 1463-9262, S. 2490–2499, doi:10.1039/C3GC42414F.
- Christophe Volkringer, Seth M. Cohen: Generating Reactive MILs: Isocyanate- and Isothiocyanate-Bearing MILs through Postsynthetic Modification. In: Angewandte Chemie International Edition. Band 49, Nr. 27, 21. Juni 2010, S. 4644–4648, doi:10.1002/anie.201001527.
- Andrea Centrone, Takuya Harada, Scott Speakman, T. Alan Hatton: Facile Synthesis of Vanadium Metal-Organic Frameworks and their Magnetic Properties. In: Small. Band 6, Nr. 15, 7. Juli 2010, S. 1598–1602, doi:10.1002/smll.201000773.
- Andrea Centrone, Takuya Harada, Scott Speakman, T. Alan Hatton: Facile Synthesis of Vanadium Metal-Organic Frameworks and their Magnetic Properties. In: Small. Band 6, Nr. 15, 7. Juli 2010, S. 1598–1602, doi:10.1002/smll.201000773.
- Alexis S. Munn, Franck Millange, Michel Frigoli, Nathalie Guillou, Clément Falaise: Iodine sequestration by thiol-modified MIL-53(Al). In: CrystEngComm. Band 18, Nr. 41, 2016, ISSN 1466-8033, S. 8108–8114, doi:10.1039/C6CE01842D.
- Shyam Biswas, Sarah Couck, Dmytro Denysenko, Asamanjoy Bhunia, Maciej Grzywa: Sorption and breathing properties of difluorinated MIL-47 and Al-MIL-53 frameworks. In: Microporous and Mesoporous Materials. Band 181, November 2013, S. 175–181, doi:10.1016/j.micromeso.2013.07.030.
- Shyam Biswas, Sarah Couck, Dmytro Denysenko, Asamanjoy Bhunia, Maciej Grzywa: Sorption and breathing properties of difluorinated MIL-47 and Al-MIL-53 frameworks. In: Microporous and Mesoporous Materials. Band 181, November 2013, S. 175–181, doi:10.1016/j.micromeso.2013.07.030.
- Claudia Zlotea, Delphine Phanon, Matjaz Mazaj, Daniela Heurtaux, Vincent Guillerm: Effect of NH2 and CF3 functionalization on the hydrogen sorption properties of MOFs. In: Dalton Transactions. Band 40, Nr. 18, 2011, ISSN 1477-9226, S. 4879, doi:10.1039/c1dt10115c.
- Karen Markey, Martin Krüger, Tomasz Seidler, Helge Reinsch, Thierry Verbiest: Emergence of Nonlinear Optical Activity by Incorporation of a Linker Carrying the p -Nitroaniline Motif in MIL-53 Frameworks. In: The Journal of Physical Chemistry C. Band 121, Nr. 45, 16. November 2017, ISSN 1932-7447, S. 25509–25519, doi:10.1021/acs.jpcc.7b09190, PMID 29170688, PMC 5694968 (freier Volltext).
- Christophe Volkringer, Thierry Loiseau, Nathalie Guillou, Gérard Férey, Mohamed Haouas: High-Throughput Aided Synthesis of the Porous Metal−Organic Framework-Type Aluminum Pyromellitate, MIL-121, with Extra Carboxylic Acid Functionalization. In: Inorganic Chemistry. Band 49, Nr. 21, November 2010, ISSN 0020-1669, S. 9852–9862, doi:10.1021/ic101128w.
- Morgane Sanselme, Jean-Marc Grenèche, Myriam Riou-Cavellec, Gérard Férey: The first ferric carboxylate with a three-dimensional hydrid open-framework (MIL-82): its synthesis, structure, magnetic behavior and study of its dehydration by Mössbauer spectroscopy. In: Solid State Sciences. Band 6, Nr. 8, August 2004, S. 853–858, doi:10.1016/j.solidstatesciences.2004.04.001.
- Christian Serre, Franck Millange, Thomas Devic, Nathalie Audebrand, Wouter Van Beek: Synthesis and structure determination of new open-framework chromium carboxylate MIL-105 or CrIII(OH)·{O2C–C6(CH3)4–CO2}·nH2O. In: Materials Research Bulletin. Band 41, Nr. 8, August 2006, S. 1550–1557, doi:10.1016/j.materresbull.2006.01.013.
- Andrea Centrone, Takuya Harada, Scott Speakman, T. Alan Hatton: Facile Synthesis of Vanadium Metal-Organic Frameworks and their Magnetic Properties. In: Small. Band 6, Nr. 15, 7. Juli 2010, S. 1598–1602, doi:10.1002/smll.201000773.
- Angiolina Comotti, Silvia Bracco, Piero Sozzani, Satoshi Horike, Ryotaro Matsuda: Nanochannels of Two Distinct Cross-Sections in a Porous Al-Based Coordination Polymer. In: Journal of the American Chemical Society. Band 130, Nr. 41, 15. Oktober 2008, ISSN 0002-7863, S. 13664–13672, doi:10.1021/ja802589u.
- Yun Liu, Jae-Hyuk Her, Anne Dailly, Anibal J. Ramirez-Cuesta, Dan A. Neumann, Craig M. Brown: Reversible Structural Transition in MIL-53 with Large Temperature Hysteresis. In: J. Am. Chem. Soc. 2008, 130, S. 11813–11818, doi:10.1021/ja803669w.
- Anne Boutin, Marie-Anne Springuel-Huet, Andrei Nossov, Antoine Gédéon, Thierry Loiseau, Christophe Volkringer, Gérard Férey, François-Xavier Coudert, Alain H. Fuchs: Breathing Transitions in MIL-53(Al) Metal-Organic Framework Upon Xenon Adsorption. In: Angewandte Chemie. 2009, 121, S. 8464–8467, doi:10.1002/ange.200903153.