JDTic
JDTic ist ein selektiver und hoch wirksamer κ-Opioidrezeptor-Antagonist[2][3][4], der in der Wissenschaft als experimenteller Wirkstoff und Ligand verwendet wird. Der über Wochen anhaltende Effekt des auch peroral wirksamen Antagonisten[5] könnte auf der Aktivierung c-Jun-N-terminaler Kinasen beruhen.[6] Verhaltenspharmakologische Studien legen nahe, dass JDTic antidepressiv, angstlösend[7] und suchtlindernd[8][9][10] wirkt.
| Strukturformel | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
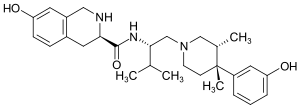 | |||||||||||||
| Allgemeines | |||||||||||||
| Name | JDTic | ||||||||||||
| Andere Namen |
(3R)-7-Hydroxy-N-[(2S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]-3-methylbutan-2-yl]-1,2,3,4-tetrahydroisochinolin-3-carboxamid | ||||||||||||
| Summenformel | C28H39N3O3 | ||||||||||||
| Externe Identifikatoren/Datenbanken | |||||||||||||
| |||||||||||||
| Eigenschaften | |||||||||||||
| Molare Masse | 465,626 g·mol−1 | ||||||||||||
| Sicherheitshinweise | |||||||||||||
| |||||||||||||
| Soweit möglich und gebräuchlich, werden SI-Einheiten verwendet. Wenn nicht anders vermerkt, gelten die angegebenen Daten bei Standardbedingungen. | |||||||||||||
Einzelnachweise
- Dieser Stoff wurde in Bezug auf seine Gefährlichkeit entweder noch nicht eingestuft oder eine verlässliche und zitierfähige Quelle hierzu wurde noch nicht gefunden.
- Thomas JB et al. (2001): Identification of the first trans-(3R,4R)- dimethyl-4-(3-hydroxyphenyl)piperidine derivative to possess highly potent and selective opioid kappa receptor antagonist activity, Journal of Medicinal Chemistry, 44(17):2687-90. PMID 11495579
- Thomas JB et al. (2003): Identification of (3R)-7-hydroxy-N-((1S)-1-[[(3R,4R)-4-(3-hydroxyphenyl)- 3,4-dimethyl-1-piperidinyl]methyl]- 2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide as a novel potent and selective opioid kappa receptor antagonist, Journal of Medicinal Chemistry, 46(14):3127-37. PMID 12825951
- Cai TB et al (2008): Synthesis and in vitro opioid receptor functional antagonism of analogues of the selective kappa opioid receptor antagonist (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)- 3,4-dimethyl- 1-piperidinyl]methyl}- 2-methylpropyl)- 1,2,3,4-tetrahydro- 3-isoquinolinecarboxamide (JDTic), Journal of Medicinal Chemistry, 51(6):1849-60. PMID 18307295
- Carroll FI et al. (2004): Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist, European Journal of Pharmacology, 501(1-3):111-9. PMID 15464069
- Bruchas MR (2007): Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase, Journal of Biological Chemistry, 282(41):29803-11. doi:10.1074/jbc.M705540200 PMID 17702750
- Knoll AT et al. (2007): Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats, Journal of Pharmacology and Experimental Therapeutics, 323(3):838-45. PMID 17823306
- Beardsley PM, Howard JL, Shelton KL, Carroll FI (2005): Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats, Psychopharmacology (Berlin), 183(1):118-26. doi:10.1007/s00213-005-0167-4 PMID 16184376
- Carroll FI, Harris LS, Aceto MD (2005): Effects of JDTic, a selective kappa-opioid receptor antagonist, on the development and expression of physical dependence on morphine using a rat continuous-infusion model, European Journal of Pharmacology, 524(1-3):89-94. PMID 16236279
- Jackson KJ, Carroll FI, Negus SS, Damaj MI (2010): Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse, Psychopharmacology (Berl), 210(2):285-94. PMID 20232057
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. The authors of the article are listed here. Additional terms may apply for the media files, click on images to show image meta data.